Origen Biomedical – The journey of a leader in cell cryopreservation
Have you ever wondered how stem cells can “hibernate” for years while maintaining their quality? The secret lies in stem cell cryopreservation solutions, a fundamental step in determining the success of every cell therapy.
Origen Biomedical, a leading biotechnology company from the United States, has established itself with international-standard stem cell cryopreservation solutions and single-use cell culture bags. It has successfully gained the trust of high-standard clients such as cancer centers, umbilical cord blood banks, and National Aeronautics and Space Administration (NASA).
Let’s explore what makes Origen Biomedical’s products the gold standard in the industry for decades.
Contents
ToggleInternational-standard stem cell cryopreservation bags from Origen Biomedical
Origen Biomedical invests up to 20% of its resources in R&D, continually improving biological cryopreservation technology. Its product development capability allows for custom orders, making Origen’s services flexible to specialized processes, even under NASA’s microgravity conditions. With a user-centered philosophy, Origen’s engineering team collaborates closely with labs to create the most suitable cell cryopreservation solutions without forcing customers to alter their procedures.
Notable achievements in R&D in 2022 include:
- CryoStore™ FLEX: The next-generation freezing bags, featuring flexible materials that reduce breakage risks.
- TissueVault™: A storage bag for solid tissues, exempt from FDA 510(k) procedures due to low-risk factors, demonstrating Origen Biomedical’s pioneering efforts in expanding cell cryopreservation to biological tissues.
With superior R&D capabilities and a customer-oriented approach, Origen Biomedical optimizes products based on real-world feedback, quickly meeting market demands, and establishing strategic partnerships in biomedical research.
International quality standards in stem cell cryopreservation
Quality is the foundation of every design and operation at Origen Biomedical. They strictly adhere to ISO 13485:2016 standards and have MDSAP certification.
The company is regularly inspected by major clients and reputable certification bodies, including the FDA (USA), CE Mark (EU), NMPA (China), and MHLW (Japan). This ensures their stem cell cryopreservation solutions meet safety and performance requirements. A prime example is the CryoStore™ freezing bags, which passed the FDA 510(k) approval process and received the CE Mark. Additionally, the PermaLife® culture bags have also gone through the 510(k) process (code K021702) for safe use in cell preservation and culture.
Origen Biomedical also quickly updates and complies with the new EU MDR regulations. Their “MDR Transition” documentation ensures that products continue to meet Europe’s stringent legal requirements after 2020.
By maintaining the highest quality standards across its biomedical equipment team, Origen Biomedical confidently emphasizes its commitment to delivering safe products that meet announced technical specifications and on-time delivery.
Origen Biomedical’s cryopreservation and cell culture product line
Origen Biomedical offers a complete range of stem cell cryopreservation and cell culture solutions:
Biological cryopreservation products
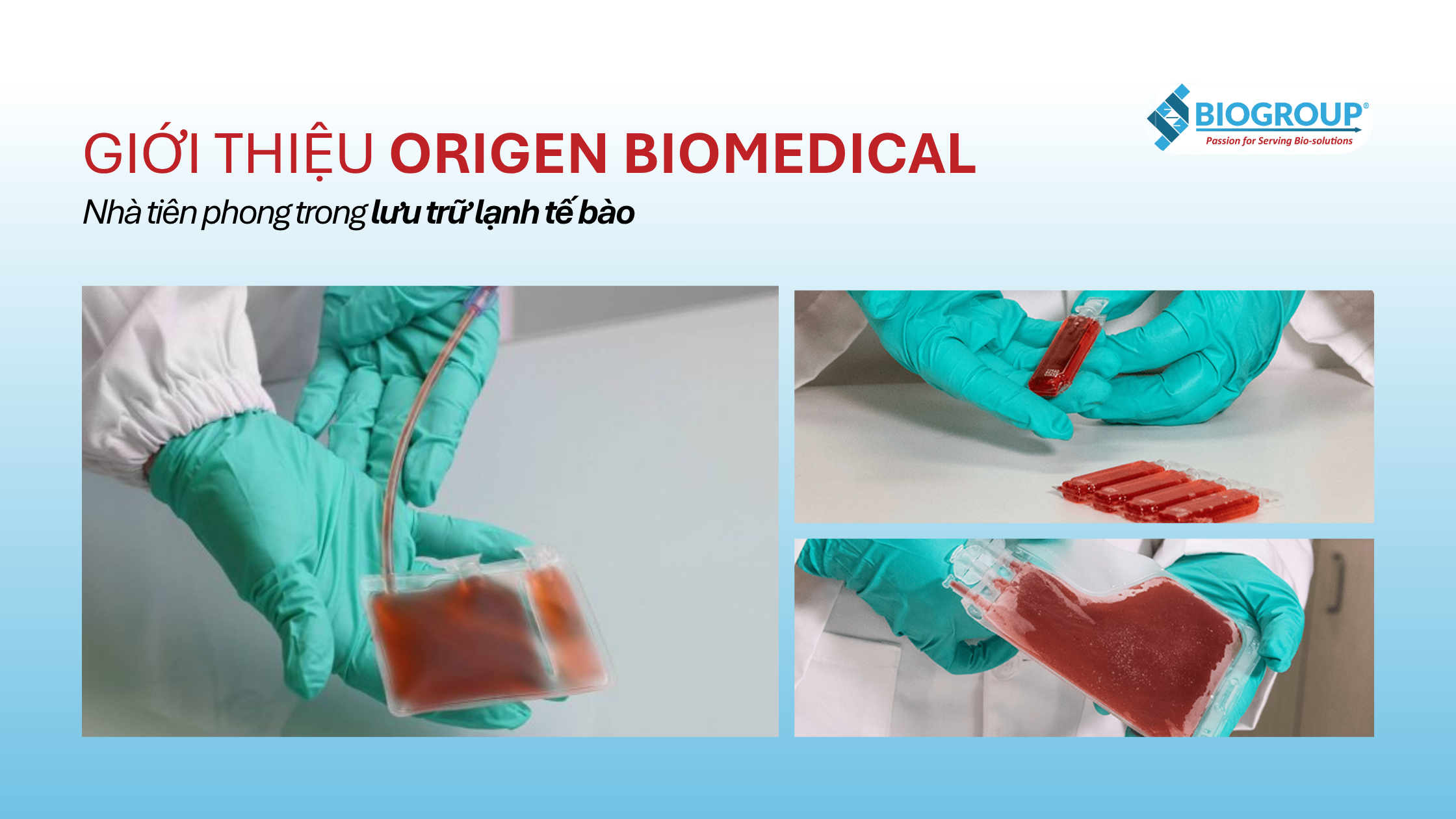
Specialty single-use cryopreservation bags under the CryoStore™ brand have been considered the “gold standard” in the industry for years. These closed systems, designed to withstand liquid nitrogen temperatures, come in various capacities (30ml—750ml). A special multi-chamber CryoStore™ bag helps segment samples without the need for complete thawing.
In addition to the main bags, Origen also offers TissueVault™, a cryopreservation bag for solid tissues, and the O-Wrap® protective wrapping for storage bags, which prevents leaks when submerged in liquid nitrogen.
Cell culture products
Origen manufactures single-use cell culture bags, including PermaLife® and Evolve™. These bags, made from pre-sterilized EVA/TPU materials, reduce the risk of contamination and integrate sampling ports, pre-installed connectors, and enhanced gas exchange.
The company also provides accessories such as Cell Connect® kits to optimize the closed-system transfer of media and cell retrieval between bags, improving the cell therapy production process.
Preservation solutions and supporting accessories
To complete the product ecosystem, Origen Biomedical offers CryoPur®, a GMP-standard DMSO preservation solution that is CE Mark certified, ensuring safety during cell infusion for patients.
The company also supplies a range of single-use accessories, including Spike Adapters, Manifold Sets, sterile syringes, and multi-sized Luer valves. These accessories enable a closed connection between bags and solutions, optimizing cell therapy production processes.
Additionally, Origen previously produced respiratory support devices for resuscitation. The company continues its strong R&D momentum and plans to expand its product catalog from 2020 to 2025 to meet the industry’s growing trends (such as CAR-T cell therapies, tissue banks, and gene therapies requiring specialized storage solutions). Hence, Origen’s product range is now considered top-notch in the niche market of cell cryopreservation and culture.
Origen Biomedical’s customer network
Origen Biomedical serves blood banks, stem cell banks, gene therapy centers, hospitals, and biological laboratories worldwide. Notable clients include:
Blood Centers of America (BCA): BCA encompasses more than 70 independent centers focused on blood, tissue, and advanced therapies, supplying over 50% of the blood in the U.S. through collaborations with hospitals, research institutions, and biotech companies. Working with BCA has significantly expanded Origen’s presence in the North American market.
MD Anderson, Mayo Clinic: These cancer research institutes and hospitals require cell storage for testing and treating cancer.
NASA: Origen has partnered with NASA to design custom cell culture bags for microgravity experiments aboard the space station.
Additionally, Origen actively participates in industry alliances and associations. Their presence at cell science conferences enables Origen to connect with experts and explore R&D collaboration opportunities, further expanding their customer base.
>> See more of Origen Biomedical’s global customer list
Origen Biomedical’s global presence
By the end of 2024, Origen Biomedical will be present in 90 countries across all continents, with major markets in North America and Europe. In the United States, the company has expanded its presence to over half of the blood banking system through partnerships like BCA. Origen has also built a strong presence in Europe by establishing distribution centers. In the Asia-Pacific region (South Korea, Southeast Asia, and particularly Japan), Origen sees a growing potential market and has actively expanded since 2020.
Vietnam is part of Origen’s emerging market, which is initially being introduced through distribution channels. Currently, Origen does not have an office or factory in Vietnam, but products like stem cell cryopreservation and cell culture are already available through official distributors. BioGroup Vietnam is introducing Origen’s products, notably the CryoStore™ FLEX bags and CryoPur® solutions. In addition to product distribution, the company provides service support and technology transfer, enhancing the customer experience and strengthening Origen’s brand in Vietnam.
Origen Biomedical’s “leading” status is based on advanced cryopreservation technology and its ability to customize cold storage solutions according to real-world needs. With a diverse product range, seamless and efficient experiences are ensured. Furthermore, Origen Biomedical continues to expand its customer base, develop new markets, and foster distribution partnerships. Biogroup Vietnam ensures the effective deployment of stem cell cryopreservation solutions. Contact us for a free consultation and to implement the optimal biological solutions tailored for you.
We’re here to help – Get your free consultation at:
- Website: https://biogroupvietnam.com/contact-us
- Email: info@biogroupvietnam.com
Categories
- Blog (53)
- New Products & Technologies (42)
- News (41)
- Recruitment (7)
- Training & Webinar (27)
- Virtual Booth (1)




